On Saturday evening, the United States health authorities have made it clear through a statement that the Moderna and Pfizer vaccines against Covid-19 for children aged five years and younger are safe. In a move, the American President Joe Biden termed this as a “monumental development” against the virus.
The United States became the first country to have approved the use of mRNA vaccines meant for children as young as 6 months. Recently on Friday, the United States Food and Drug Administration (FDA) have authorized the emergence use of these vaccines for young children – previously, the age limit was set to at least be five years old. On Saturday, the country’s leading public health agency, the United States Centers for Disease Control and Prevention (CDC) have also approved the use of vaccines for young children.
Rochelle Walensky, the CDC Director, stated on Saturday, “We know millions of parents and caregivers are eager to get their young children vaccinated, and with today’s decision, they can.” As the FDA gave a clearance on the use of these vaccines, the US government began the distribution of million doses across the country.
Also Read: SpaceX terminate employees on breach attempted against CEO Elon Musk
The United States President Joe Biden stated that parents can begin taking appointments from next month at the earliest and get their young children vaccinated against the virus. The vaccination can be done in hospitals, pharmacies, clinics, and doctor’s chambers.
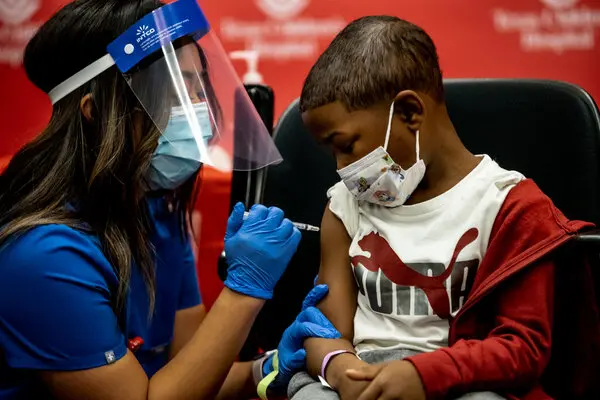
In the same statement released on Saturday, Biden claimed the vaccines to be “safe (and) highly effective” and further added that “for parents all over the country, this is a day of relief and celebration.” In the coming weeks, higher number of doses will be shipped out and “every parent who wants a vaccine will be able to get one” he said.
The Moderna vaccine against Covid-19 is touted into two doses which are a month apart – it will be available for children aged 6 months to five years in doses of 25 micrograms. This is half the dose given to children aged six, and quarter of those given to 12 years and beyond.
Bio-Tech’s Pfizer vaccine against Covid-19 is available for children aged 6 months to four years, and is meant to be given in doses comprising three micrograms per shot. This comprises of 1/10th of what is given to adults.
Children taking Pfizer shot will receive it in three shots; the first two doses will be three weeks apart, and the third dose will be given after eight weeks. This is a precautionary move to ensure children’s safety in the first few months.
Also Read: Paul Walker gets featured on the Hollywood Walk of Fame; fans pour all the love
Pfizer’s side effect, in comparison to Moderna, during the drug trial was milder. About 1/3rd of children receiving jabs of Moderna vaccine developed fevers, which lasted for a day or two during the drug trial. As per reports, approximately 20 million children in the United States are now eligible for both the vaccines.